PN-Heterocycles
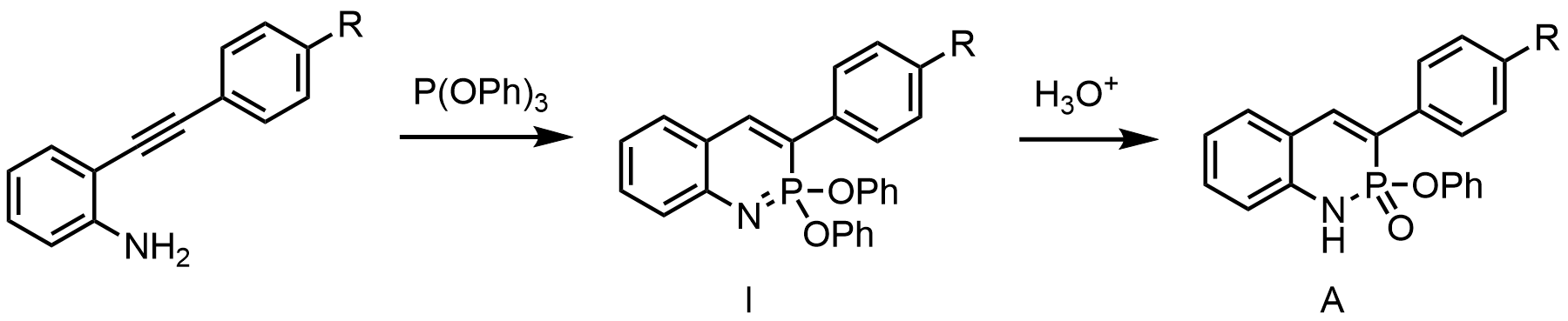
In summer 2014 graduate student Chris Vonnegut accidentally discovered that treatment of an ortho-(arylethynyl)aniline with triphenylphosphite gave a class of heterocycles with contiguous nitrogen and phosphorus atoms. As shown in the figure below, the initial product of the cyclization reaction is the phosphaquinoline form (imidate, I). Subsequent hydrolysis affords the phosphaquinolinone form (amidate, A).
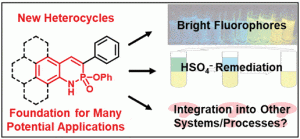
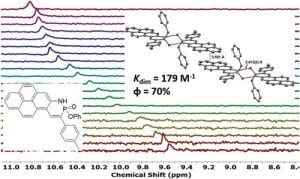
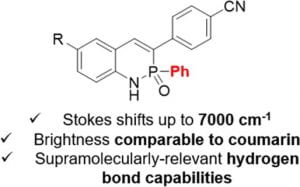
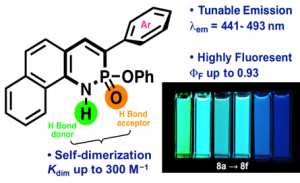
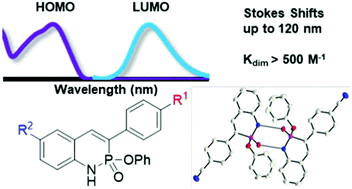
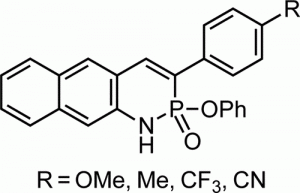
Since Chris’ discovery, we have been studying the structural and optical properties of these rarely-accessed scaffolds. The heterocyclic amidate core is structurally similar to coumarin and carbostyril, two well-studied fluorophores, with the difference being that the carbonyl in carbostyril has been replaced with an isolobal phosphonyl group. The structural similarities of the phosphaquinolinone scaffold to coumarin and carbostyril also extend to other features, including the highly tunable and substitution-dependent photophysical properties as well as the homodimerization seen in lactams.
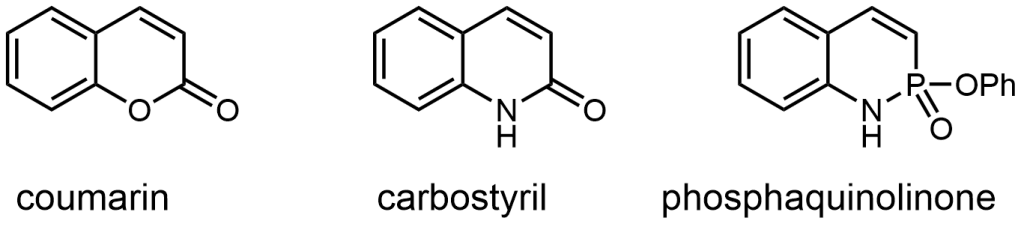
These phosphorous-nitrogen heterocycles, which are properly named as azaphosphinines, boast the benefit of containing a chiral center on the phosphorus atom (each dimer contains one R- and one S-enantiomer) and enhanced homodimerization due to the highly polarized P=O bond. In addition, the tetrahedral center facilitates the selective binding of hydrogen sulfate, a typically difficult anion to host due to its geometry and combination of both hydrogen-bond donor and acceptors
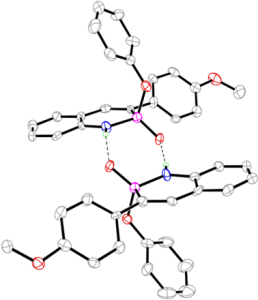
Recent and ongoing work has focused on larger acene-expanded aniline derivatives as well as the inclusion of additional heteroatoms within the backbone to further modulate the photophysical and supramolecular capabilities of these fascinating heterocycles.
Relevant Publications
Azaphosphinines and Their Derivatives
J. N. McNeill, J. P. Bard, D. W. Johnson and M. M. Haley, Chem. Soc. Rev. 2023, 52, 8599-8634.
DOI: 10.1039/D3CS00737E
2‐λ5‐Phosphaquinolin‐2‐ones as Non-Cytotoxic, Targetable, and pH-Stable Fluorophores
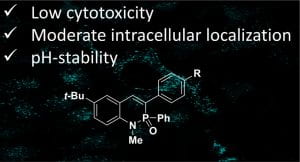
J. P. Bard, S. G. Bolton, H. J. Howard, J. N. McNeill, L. N. Zakharov, D. W. Johnson, M. D. Pluth and M. M. Haley, J. Org. Chem. 2023, 88, 15516-15522.
DOI: 10.1021/acs.joc.3c01927
Impact of Internal Charge Transfer on the Photophysical Properties of Pyridine-Fused Phosphorus-Nitrogen Heterocycles
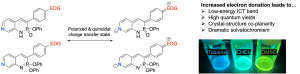
J. N. McNeill, M. A. Kascoutas, L. J. Karas, L. N. Zakharov, J. I.-C. Wu, D. W. Johnson and M. M. Haley, Chem. Eur. J. 2023, 29, e202203918.
DOI:10.1002/chem.202203918
Controlling Tautomerization in Pyridine-Fused Phosphorus-Nitrogen Heterocycles
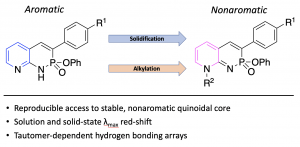
J. N. McNeill, L. J. Karas, J. P. Bard, K. Fabrizio, L. N. Zakharov, S. N. MacMillan, C. K. Brozek, J. I. Wu, D. W. Johnson and M. M. Haley, Chem. Eur. J. 2022, 28, e202200472.
DOI: 10.1002/chem.202200472
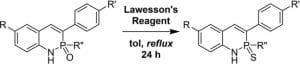
Thionation of the 2‐λ5‐Phosphaquinolin‐2‐one Scaffold with Lawesson’s Reagent
Bard, J. P.; McNeill, J. N.; Warren, G. I.; Zakharov, L. N.; Johnson, D. W.; Haley, M. M. Isr. J. Chem. 2021, 61, 217-221.
DOI: 10.1002/ijch.202000085
Bumpy Roads Lead to Beautiful Places: The Twists and Turns in Developing a New Class of PN-Heterocycles
Bard, J. P.; Johnson, D. W.; Haley, M. M. Synlett 2020, 31, 1862-1867.
DOI: 10.1055/s-0040-1707168
A highly fluorescent PN-heterocycle-fused pyrene derivative with strong self-dimerisation through hydrogen bonding
Bard, J. P.; Mancuso, J. L.; Deng, C.-L.; Zakharov, L. N.; Johnson, D. W.; Haley, M. M. Supramol. Chem. 2020, 32, 49-55.
DOI: 10.1080/10610278.2019.1687896
Amplification of the Quantum Yields of 2-λ5-Phosphaquinolin-2-ones through Phosphorus Center Modification
Bard, J. P.; Bates, H. J.; Deng, C.-L.; Zakharov, L. N.; Johnson, D. W.; Haley, M. M. J. Org. Chem. 2019, 85, 85-91.
DOI: 10.1021/acs.joc.9b02132
PN-Containing Pyrene Derivatives: Synthesis, Structure, and Photophysical Properties
Deng, C.-L.; Bard, J. P.; Zakharov, L. N.; Johnson, D. W.; Haley, M. M. Org. Lett. 2019, 21, 6427-6431.
DOI: 10.1021/acs.orglett.9b02332
Naphtho[2,1-e]-1,2-azaphosphorine-2-oxide Derivatives: Synthesis, Optoelectronic Properties, and Self-Dimerization Phenomena
Deng, C.-L.; Bard, J. P.; Zakharov, L. N.; Johnson, D. W.; Haley, M. M. J. Org. Chem. 2019, 84, 8131-8139.
DOI: 10.1021/acs.joc.9b00994
Exploiting the Hydrogen Bond Donor/Acceptor Properties of PN-Heterocycles: Selective Anion Receptors for Hydrogen Sulfate
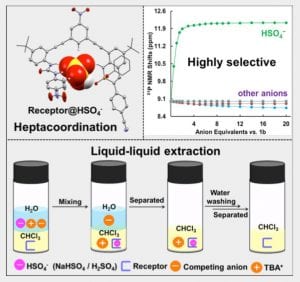
C.-L. Deng, J. P. Bard, J. A. Lohrman, J. E. Barker, L. N. Zakharov, D. W. Johnson and M. M. Haley, Angew. Chem. Int. Ed. 2019, 58, 3934-3938.
DOI: 10.1002/anie.201814431
Synthesis, photophysical properties, and self-dimerization studies of 2-λ5-phosphaquinolin-2-ones
Bard, J. P.; Deng, C. L.; Richardson, H. C.; Odulio, J. M.; Barker, J. E.; Zakharov, L. N.; Cheong, P. H.-Y.; Johnson, D. W.; Haley, M. M. Org. Chem. Front. 2019, 6, 1257-1265.
DOI: 10.1039/C9QO00199A
Synthesis and Properties of Naphtho[2,3-e]-1,2-azaphosphorine 2-Oxides: PN-Anthracene Analogues
Takaesu, N. A.; Ohta, E.; Zakharov, L. N.; Johnson, D. W.; Haley, M. M. Organometallics 2017, 36, 2491-2493.
DOI: 10.1021/acs.organomet.7b00281
Facile Synthesis and Properties of 2-λ5-Phosphaquinolines and 2-λ5-Phosphaquinolinones
Vonnegut, C. L.; Shonkwiler, A. M.; Khalifa, M. K.; Zakharov, L. N.; Johnson, D. W.; Haley, M. M. Angew. Chem. Int. Ed. 2015, 54, 13318-13322.
DOI: 10.1002/anie.201507696