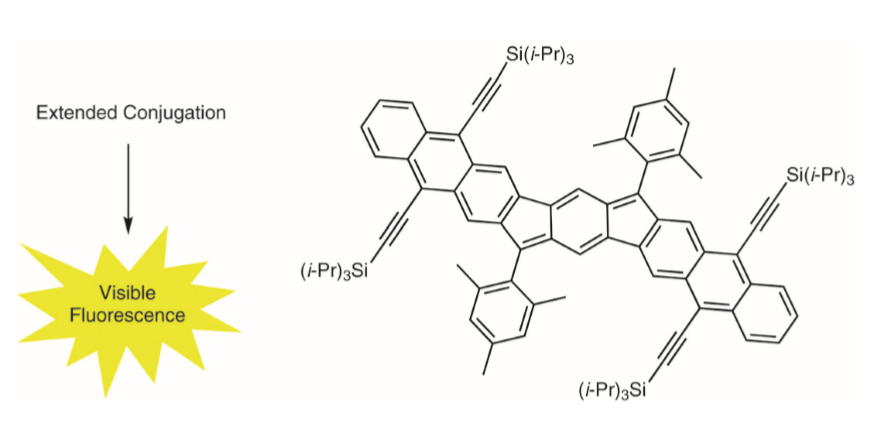
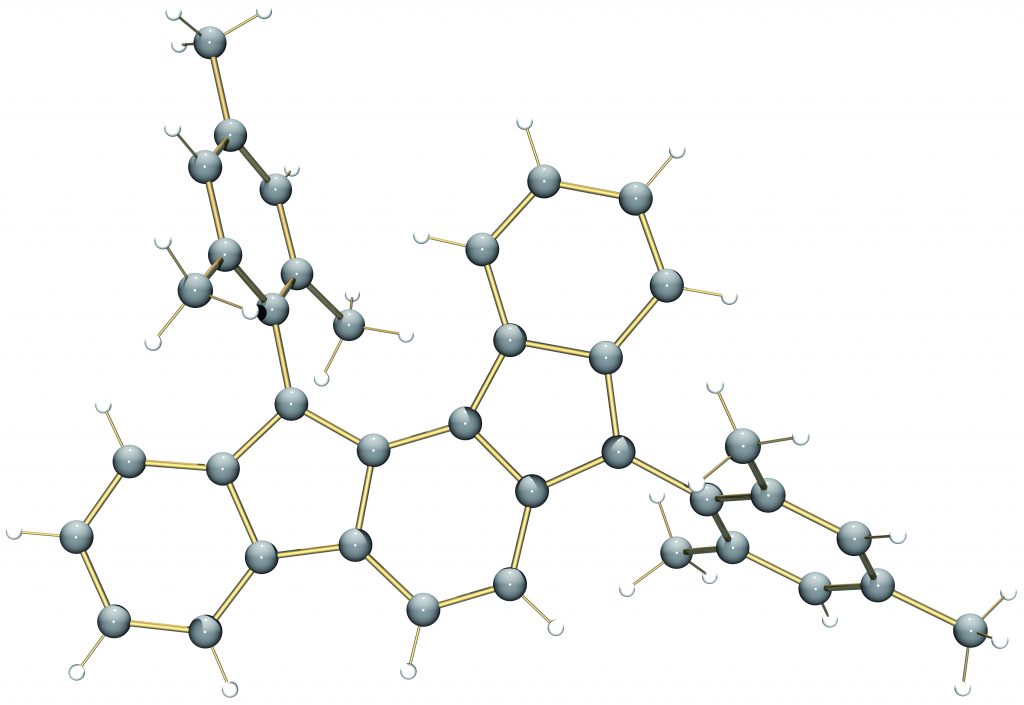
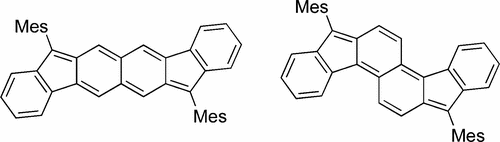
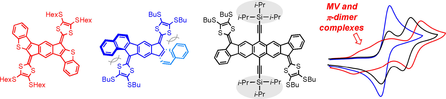
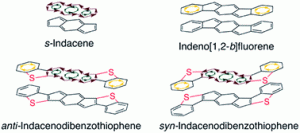
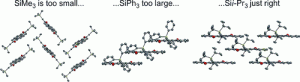
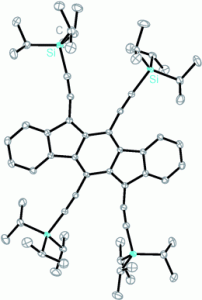
Indenofluorene Scaffolds
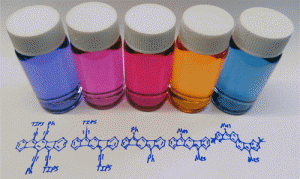
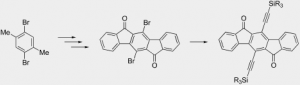
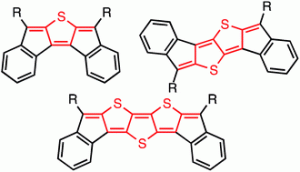
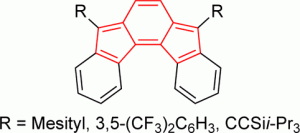
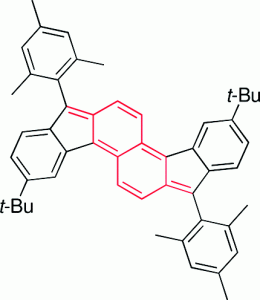
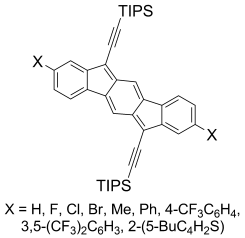
Polycyclic hydrocarbons that possess extended π-conjugation are of tremendous interest due to their potential use in optical and 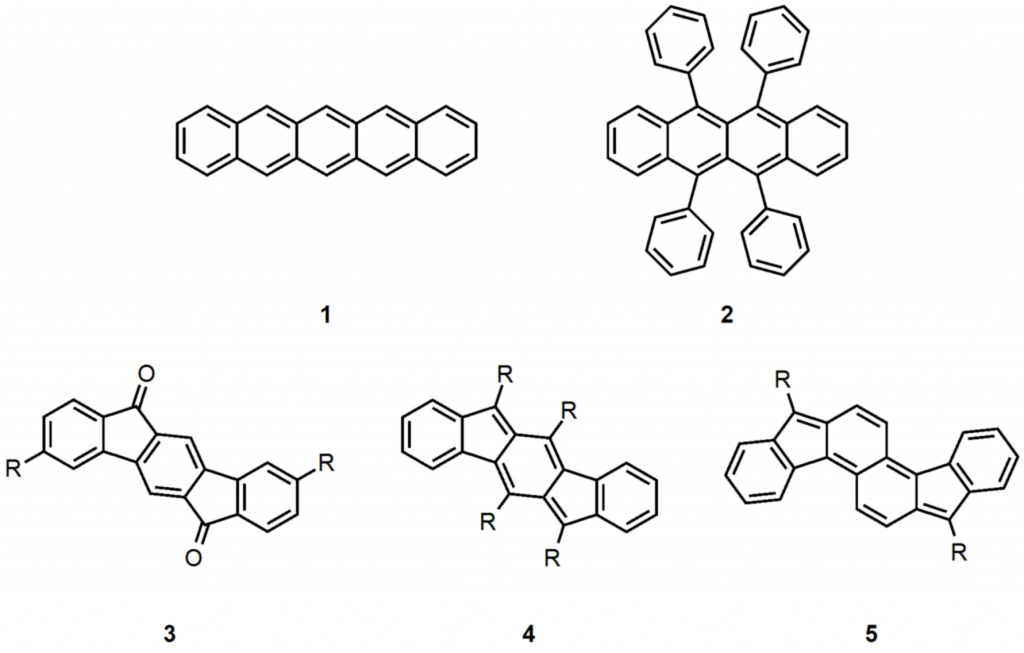 electronic device applications. While a majority of studies have focused on acenes and their derivatives (for example, compounds 1-2), these systems are susceptible to degradation due to environmental effects, through oxidative or photolytic pathways; thus, there is a pressing need for alternative, acene-like topologies. Current research in the lab is focused on molecules based on or inspired by the indenofluorene (IF) skeleton, such as compounds 3-5. Over the last few years, we have adapted and/or developed general methods for the assembly of a variety of fully conjugated IF derivatives and initiated exploration of their materials properties. We have shown that IFs can be prepared in gram quantities with good overall yields and excellent purity using methodologies amenable to large-scale production.
electronic device applications. While a majority of studies have focused on acenes and their derivatives (for example, compounds 1-2), these systems are susceptible to degradation due to environmental effects, through oxidative or photolytic pathways; thus, there is a pressing need for alternative, acene-like topologies. Current research in the lab is focused on molecules based on or inspired by the indenofluorene (IF) skeleton, such as compounds 3-5. Over the last few years, we have adapted and/or developed general methods for the assembly of a variety of fully conjugated IF derivatives and initiated exploration of their materials properties. We have shown that IFs can be prepared in gram quantities with good overall yields and excellent purity using methodologies amenable to large-scale production.
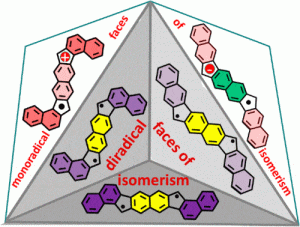
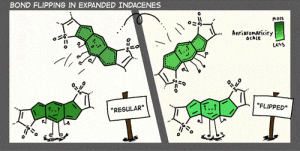
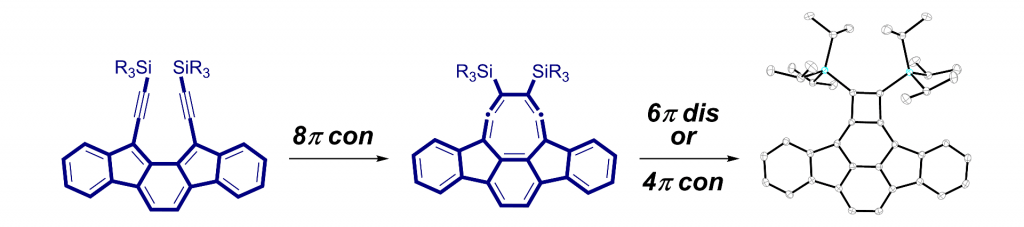
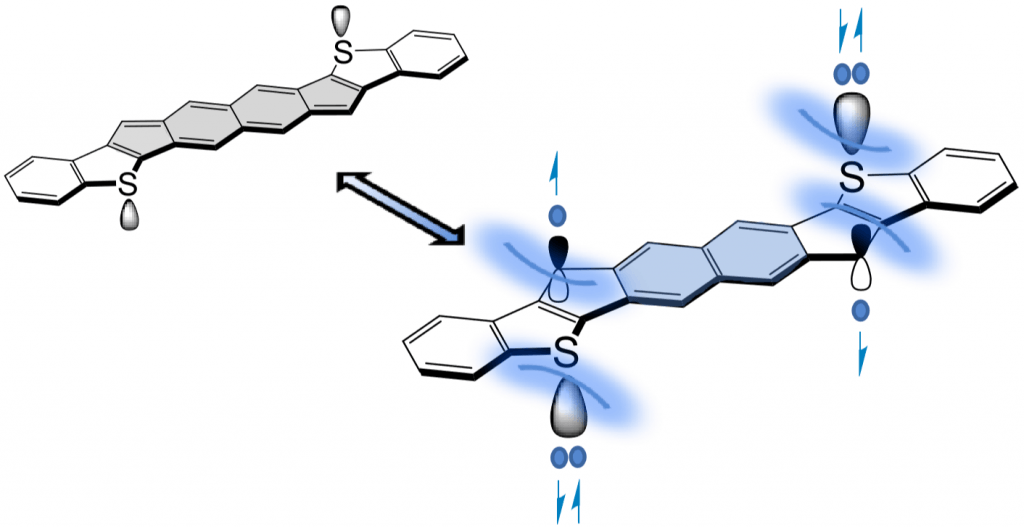
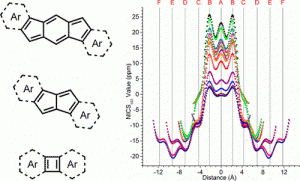
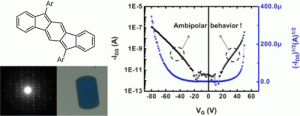
In addition to being challenging synthetic targets, IFs help to provide answers to fundamental questions about structure, bonding, and reactivity in expanded, conjugated structures. They also have the potential to act as rigid, planar, electron-accepting cores for the formation of advanced materials with novel electronic properties. We are exploiting the materials potential of IFs via a combined experimental and theoretical approach, with an emphasis toward the use of indenofluorenes as organic semiconductors in devices. Importantly, we have demonstrated that single crystals, as well OFETs of IFs can serve as an active layer in an organic field-effect transistors that exhibit ambipolar behavior.
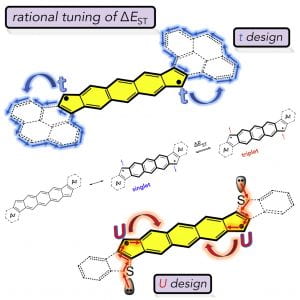
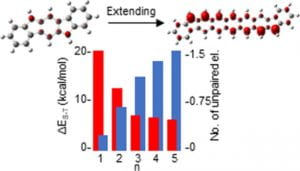
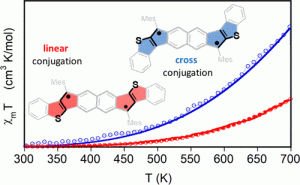
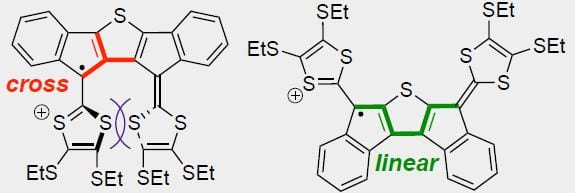
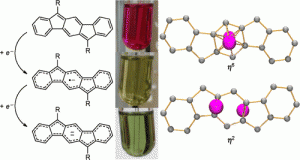
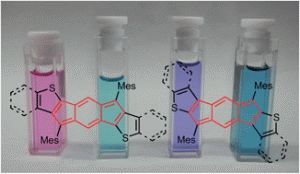
Recent work in the group has shown that we can access open-shell variants and tune the singlet-triplet energy gap of the diradicaloid species.
Selected Publications
“Effects Of Benzoheterocyclic Annelation On The s-Indacene Core: A Computational Analysis”

Warren, G. I.; Młodzikowska-Pieńko, K.; Jalife, S.; Demachkie, I. S.; Wu, J. I.; Haley, M. M.; Gershoni-Poranne, R. Chem Sci. 2025, 16, 575-583.
DOI:https://doi.org/10.1039/D4SC06812B
“Intramolecular Charge Transfer In Antiaromatic Donor/Acceptor-Fused s-Indacenes”

Demachkie, I. S.; Miller, M. P.; Warren, G. I.; Barker, J. E.; Strand, E. T.; Zakharov, L. N.; Haley, M. M. Angew. Chem. Int. Ed. 2025, 64, e202420989.
DOI:https://doi.org/10.1002/anie.202420989
“Extreme Anomalous Conductance Enhancement In Neutral Diradical Acene-Like Molecular Junctions”

Lawson, B.; Vidal Jr., E.; Luna, S.; Haley, M.M.; Kamanetska, M. ACS Nano 2024, 18, 29059-29066.
DOI:10.1021/acsnano.4c10183
“Probing The Influence Of Alkyne Substitution On The Electronic And Magnetic Properties Of Diindeno[1,2-b;1′,2′-i]anthracenes”

Vidal Jr., E.; Zakharov, L.N.; Gomez-Garcia, C.J.; Haley, M.M. J. Org. Chem. 2024, 89, 14515-14519.
DOI:10.1021/acs.joc.4c01500
“Computational Comparison Of Paratropicity Trends In Antiaromatic s-indacene Derivatives: Does The Functional ‘Make All The Difference’?”

Miller, M.P.; Haley, M.M. J. Phys. Org. Chem. 2025, 38, e4648.
DOI:10.1002/poc.4648
“From Solution To Surface: Persistence Of The Diradical Character Of A Diindenoanthracene Derivative On A Metallic Substrate”
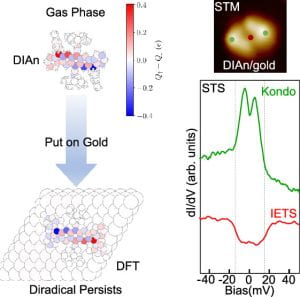
Hieulle, J.; Garcia Fernandez, C.; Friedrich, N.; Vegliante, A.; Sanz, S.; Sanchez-Portal, D.; Haley, M.M.; Casado, Juan.; Frederiksen, T.; Ignacio Pascual, J. J. Phys. Chem. Lett. 2023, 14, 11506-11512.
DOI: 10.1021/acs.jpclett.3c02401
“Tetra-tert-butyl-s-Indacene Is A Bond Localized C2h Structure And A Challenge For Computational Chemistry”
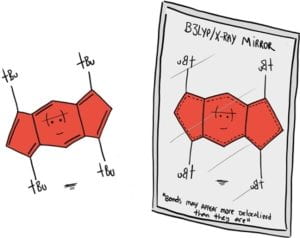
Karas, L.S.; Jalife, S.; Viesser, R.V.; Soares, J.V.; Haley, M.M.; Wu, J.I. Angew. Chem. Int. Ed. 2023, 62, e202307379.
DOI: 10.1002/anie.202307379
See Team Profile: DOI: 10.1002/anie.202317561
“Comparison of Antiaromatic Properties in a Series of Structurally Isomeric Naphthothiophene-fused s-Indacenes”
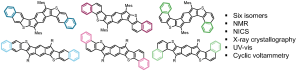
Warren, G.I.; Zocchi, L.J.; Zakharov, L.N.; Haley, M.M. Chem. Eur. J. 2023, e202301153.
DOI: 10.1002/chem.202301153
“Orbital Nature of Carboionic Monoradicals Made from Diradicals”
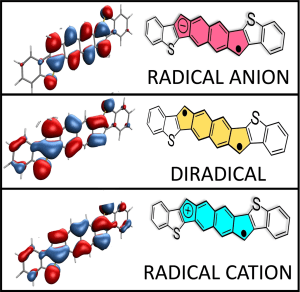
A. Cardenas Valdivia, Y. Dai, F. Rambaldi, Z. Zhou, Y. Zhu, Z. Wei, J. E. Barker, J. J. Dressler, M. A. Petrukina, M. M. Haley, F. Negri and J. Casado, Chem. Eur. J.2023, 29, e202300388.
DOI: 10.1002/chem.202300388
“Polycyclic Hydrocarbons from [4n]Annulenes: Correlation versus Hybridization Forces in the Formation of Diradicaloids”

Quintero, S.M.; Haley, M.M.; Ketesz, M.; Casado, J. Angew. Chem. Int. Ed. 2022, 61, e202209138.
DOI: 10.1002/anie.202209138
“A Tale of Two Isomers: Enhanced Antiaromaticity/Diradical Character versus Deleterious Ring-Opening of Benzofuran-fused s-Indacenes and Dicyclopenta[b,g]naphthalenes”
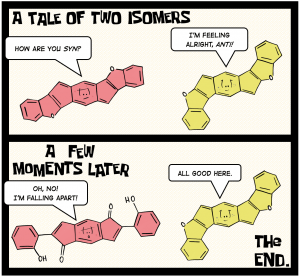
Barker, J. E.; Price, T. W.; Karas, L. J.;Kishi, R.;MacMillan, S. N.; Zakharov, L. N.; Gómez-García, C. J.; Wu, J. I.; Nakano, M.; Haley, M. M. Angew. Chem. Int. Ed.2021, 60, 22385-22392.
DOI: 10.1002/anie.202107855
“OCELOT: An Infrastructure for Data-Driven Research to Discover and Design Crystalline Organic Semiconductors”

Ai, Q.; Bhat, V.; Ryno, S. M.; Jarolimek, K.; Sornberger, P.; Smith, A.; Haley, M. M.; Anthony, J. E.; Risko, C. J. Chem. Phys.2021, 154, 174705.
DOI: 10.1063/5.0048714
“Enhancing the Antiaromaticity of s-Indacene Through Naphthothiophene Fusion”
Warren, G. I.; Barker, J. E.; Zakharov, L. N.; Haley, M. M. Org. Lett. 2021, 23, 5012-5017.
DOI: 10.1021/acs.orglett.1c01514
“Monoradicals and Diradicals of Dibenzofluoreno[3,2-b]fluorene Isomers: Mechanisms of Electronic Delocalization”
Hayashi, H.; Barker, J. E.; Valdivia, A. C.; Kishi, R.; MacMillan, S. N.; Gómez-García, C. J.; Miyauchi, H.; Nakamura, Y.; Nakano, M.; Kato, S.-I.; Haley, M. M.; Casado, J. J. Am. Chem. Soc. 2020 142, 20444-20455.
DOI: 10.1021/jacs.0c09588
“Late-stage Modification of Electronic properties of Antiaromatic and Diradicaloid Indeno[1,2-b]fluorene Analogues via Sulfur Oxidation”
Dressler, J. J.; Barker, J. E.; Karas, L. J.; Hashimoto, H.; Kishi, R.; Zakharov, L. N. MacMillan, S. N.; Gómez-García, C. J.; Nakano, M.; Wu, J. I.; Haley, M. M. J. Org. Chem. 2020 85, 10846-10857.
DOI: 10.1021/acs.joc.0c01387
“Learning how to fine-tune diradical properties by structure refinement”
Dressler, J. J.; Haley, M. M. J. Phys. Org. Chem. 2020, 33, e4114.
DOI: 10.1002/poc.4114
“Diindenoanthracene Diradicaloids Enable Rational, Incremental Tuning of Their Singlet-Triplet Energy Gaps”
Dressler, J. J.; Valdivia, A. C.; Kishi, R.; Rudebusch, G. E.; Ventura, A. M.; Chastain, B. E.; Gómez-García, C. J.; Zakharov, L. N.; Nakano, M.; Casado, J.; Haley, M. M. Chem 2020, 6, 1353-1368.
DOI: 10.1016/j.chempr.2020.02.010
“Interplay of Biradicaloid Character and Singlet/Triplet Energy Splitting for cis-/trans-Diindenoacenes and Related Benzothiophene-Capped Oligomers as Revealed by Extended Multireference Calculations”
Nieman, R.; Silva, N. J.; Aquino, A. J. A.; Haley, M. M.; Lischka, H. J. Org. Chem. 2020, 85, 3664-3675.
DOI: 10.1021/acs.joc.9b03308
“Molecule Isomerism Modulates the Diradical Properties of Stable Singlet Diradicaloids”
Barker, J. E.; Dressler, J. J.; Valdivia, A. C.; Kishi, R.; Strand, E. T.; Zakharov, L .N; MacMillan, S. N.; Gómez-García, C. J.; Nakano, M.; Casado, J.; Haley, M. M. J. Am. Chem. Soc. 2020, 142, 1548-1555.
DOI: 10.1021/jacs.9b11898
“Organic Semiconductors Derived from Dinaphtho-Fused s-Indacenes: How Molecular Structure and Film Morphology Influence Thin-Film Transistor Performance”
Zeidell, A. M.; Jennings, L.; Frederickson, C. K.; Ai, Q.; Dressler, J. J.; Zakharov, L. N.; Risko, C.; Haley, M. M.; Jurchescu, O. D. Chem. Mater. 2019, 31, 6962-6970.
DOI: 10.1021/acs.chemmater.9b01436
Serendipitous Rediscovery of the Facile Cyclization of Z,Z-3,5-Octadiene-1,7-diyne Derivatives to Afford Stable, Substituted Naphthocyclobutadienes”
Barker, J. E.; Kodama, T.; Song, M. K.; Frederickson, C. K.; Jousselin-Oba, T.; Zakharov, L. N.; Marrot, J.; Frigoli, M.; Johnson, R. P.; Haley, M. M. ChemPlusChem 2019, 84, 665-672.
DOI: 10.1002/cplu.201800605
Tuning Redox Properties and Self-Assembly of Thienoacene-Extended Tetrathiafulvalenes.”
Andersen, C. L.; Zalibera, M.; Lušpai, K.; Christensen, M. A.; Darvasiová, D; Lukes, B.; Rapta, P.; Haley, M. M.; Hammerich, O.; Nielsen, M. B. ChemPlusChem 2019, 84, 1279-1287.
DOI: 10.1002/cplu.201800626
Synthesis and Properties of Benzo-Fused Indeno[2,1-c]fluorenes
Jousselin-Oba, T.; Deal, P. E.; Fix, A. G.; Frederickson, C. K.; Vonnegut, C. L.; Yassar, Abderrahim, Y.; Zakharov, L. N.; Frigoli, M.; Haley, M. M. Chem. Asian J. 2019, 14, 1737-1744.
DOI: 10.1002/asia.201801684
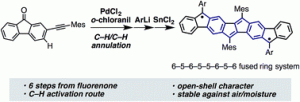
Thiophene and its sulfur inhibit indenoindenodibenzothiophene diradicals from low-energy lying thermal triplets
Dressler, J. J.; Teraoka, M; Espejo, G. L.; Kishi, R.; Takamuku, S.; Gómez-García, C. J.; Zakharov, L. N.; Nakano, M.; Casado, J.; Haley, M. M. Nat. Chem. 2018, 10, 1134-1140.
DOI: 10.1038/s41557-018-0133-5
Synthesis and Characterization of a Fluorescent Dianthracenoindacene
Frederickson, C. K.; Barker, J. E.; Dressler, J. J.; Zhou, Z.; Hanks, E. R.; Bard, J. P.; Zakharov, L. N.; Petrukhina, M. A.; Haley, M. M. Synlett 2018 29, 2562-2566.
DOI: 10.1055/s-0037-1610280
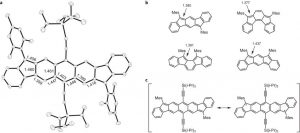
Synthesis of 7,12-Dimesitylindeno[1,2-a]fluorene, the Sole Unknown Indenofluorene Regioisomer, and Crystallographic Characterization via Its Dianion
Dressler, J. J.; Zhou, Z.; Marshall, J. L.; Kishi, R.; Takamuku, S.; Wei, Z.; Spisak, S.; Nakano, M.; Petrukhina, M. A.; Haley, M. M. Angew. Chem. Int. Ed. 2017, 48, 15363–15367.
DOI: 10.1002/anie.201709282
Synthesis and Properties of Quinoidal Fluorenofluorenes
Barker, J. E.; Frederickson, C. K.; Jones, M. H.; Zakharov, L. N.; Haley, M. M. Org. Lett. 2017, 19, 5312-5315.
DOI: 10.1021/acs.orglett.7b02605
Expanded Indacene-Tetrathiafulvalene Scaffolds: Structural Implications for Redox Properties and Association Behavior
Petersen, J. F.; Frederickson, C. K.; Marshall, J. L.; Rudebusch, G. E.; Zakharov, L. N.; Hammerich, O.; Haley, M. M.; Nielsen, M. B. Chem. Eur. J. 2017, 23, 13120-13130.
DOI: 10.1002/chem.201702347
Explorations of the Indenofluorenes and Expanded Quinoidal Analogues
Frederickson, C. K.; Rose, B. D.; Haley, M. M. Acc. Chem. Res. 2017, 50, 977-987.
DOI: 10.1021/acs.accounts.7b00004
Modulating Paratropicity Strength in Diareno-Fused Antiaromatics
Frederickson, C. K.; Zakharov, L. N.; Haley, M. M. J. Am. Chem. Soc. 2016, 138, 16827-16838.
DOI: 10.1021/jacs.6b11397
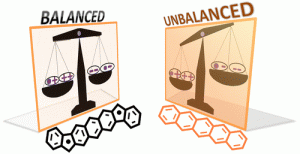
A Biradical Balancing Act: Redox Amphoterism in a Diindenoanthracene Derivative Results from Quinoidal Acceptor and Aromatic Donor Motifs
Rudebusch, G. E.; Espejo, G. L.; Zafra, J. L.; Peña-Alvarez, M.; Spisak, S. N.; Fukuda, K.; Wei, Z.; Nakano, M.; Petrikhina, M. A.; Casado, J.; Haley, M. M. J. Am. Chem. Soc. 2016, 138, 12648-12654.
DOI: 10.1021/jacs.6b07882
Diindeno-fusion of an anthracene as a design strategy for stable organic biradicals
Rudebusch, G. E.; Zafra, J. L.; Jorner, K.; Fukuda, K.; Marshall. J. L.; Arrechea-Marcos, I.; Espejo, G. L.; Ortiz, R. P; Gómez-García, C. J.; Zakharov, L. N.; Nakano, M.; Ottoson, H.; Casado, J.; Haley, M. M. Nat. Chem. 2016, 8, 753-759.
DOI: 10.1038/nchem.2518
Indacenodibenzothiophenes: synthesis, optoelectronic properties and materials applications of molecules with strong antiaromatic character
Marshall. J. L.; Uchida, K.; Frederickson, C. K.; Schütt, C.; Zeidell, A. M.; Goetz, K. P.; Finn, T. W.; Jarolimek, K.; Zakharov, L. N.; Risko, C.; Herges, R.; Jurchescu, O. D.; Haley, M. M. Chem. Sci. 2016, 7, 5547-5558.
DOI: 10.1039/C6SC00950F
Synthesis and Characterization of Two Unsymmetrical Indenofluorene Analogues: Benzo[5,6]-s-indaceno[1,2-b]thiophene and Benzo[5,6]-s-indaceno[2,1-b]thiophene
Marshall. J. L.; O’Neal, N. J.; Zakharov, L. N.; Haley, M. M. J. Org. Chem. 2016, 81, 3674-3680.
DOI: 10.1021/acs.joc.6b00340
Synthesis of Open-Shell Ladder π-Systems by Catalytic C–H Annulation of Diarylacetylenes
Maekawa, T.; Ueno, H.; Segawa, Y.; Haley, M. M.; Itami, K. Chem. Sci. 2016, 7, 650-654.
DOI: 10.1039/C5SC03391H
Synthesis and properties of fully conjugated indacenediselenophene and diindenoselenophene derivatives
Marshall. J. L.; Rudebusch, G. E.; Vonnegut, C. L.; Zakharov, L. N.; Haley, M. M. Tetrahedron Lett. 2015, 56, 3235-3239.
DOI: 10.1016/j.tetlet.2014.12.096
Synthesis and Optoelectronic Properties of Indeno[1,2-b]fluorene-6,12-dione Donor–Acceptor–Donor Triads
Frederickson, C. K.; Haley, M. M. J. Org. Chem. 2014, 79, 11241-11245.
DOI: 10.1021/jo502009p
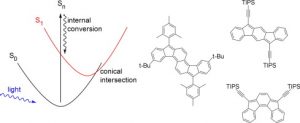
Unusually short excited state lifetimes of indenofluorene and fluorenofluorene derivatives result from a conical intersection
Rose, B. D.; Shoerb, L. E.; Wasielewski, M. R.; Haley, M. M. Chem. Phys. Lett. 2014, 616-617, 137-141.
DOI: 10.1016/j.cplett.2014.10.031
Scalable synthesis of 5,11-diethynylated indeno[1,2-b]fluorene-6,12-diones and exploration of their solid state packing
Rose, B. D.; Santa Maria, P. J.; Fix, A.G.; Vonnegut, C. L.; Zakharov, L.; Parkin, S. R.; Haley, M. M. Beilstein J. Org. Chem. 2014, 10, 2122–2130.
DOI: 10.3762/bjoc.10.219
Quinoidal diindenothienoacenes: Synthesis and properties of new functional organic materials
Rudebusch, G. E.; Fix, A. G.; Henthorn, H. A.; Vonnegut, C. L.; Zakharov, L.; Haley, M. M. Chem. Sci. 2014, 5, 3627-3633.
DOI: 10.1039/C4SC01432D
Experimental and Computational Studies of the Neutral and Reduced States of Indeno[1,2-b]fluorene
Rose, B. D.; Sumner, N. J.; Filatov, A. S.; Peters, S. J.; Zakharov, L. N.; Petrukhina, M. A.; Haley, M. M. J. Am. Chem. Soc. 2014, 136, 9181–9189.
DOI: 10.1021/ja503870z
Synthesis and properties of fully-conjugated indacenedithiophenes
Young, B. S.; Chase, D. T.; Marshall, J. L.; Vonnegut, C. L.; Zakharov, L. N.; Haley, M. M. Chem. Sci. 2014, 5, 1008-1014.
DOI: 10.1039/C3SC53181C
Indeno[2,1-c]fluorene: A New Electron-Accepting Scaffold for Organic Electronics
Fix, A. G.; Deal, P. E.; Vonnegut, C. L.; Rose, B. D.; Zakharov, L. N.; Haley, M. M. Org. Lett. 2013, 15, 1362–1365.
DOI: 10.1021/ol400318z
6,12-Diarylindeno[1,2-b]fluorenes: Syntheses, Photophysics, and Ambipolar OFETs
Chase, D. T.; Fix, A. G.; Kang, S. J.; Rose, B. D.; Weber, C. D.; Zhong, Y.; Zakharov, L. N.; Lonergan, M. C.; Nuckolls, C.; Haley, M. M. J. Am. Chem. Soc. 2012, 134, 10349–10352.
DOI: 10.1021/ja303402p
Fluoreno[4,3-c]fluorene: A Closed-Shell, Fully Conjugated Hydrocarbon
Rose, B. D.; Vonnegut, C. L.; Zakharov, L. N.; Haley, M. M. Org. Lett. 2012, 14, 2426–2429.
DOI: 10.1021/ol300942z
Electron-Accepting 6,12-Diethynylindeno[1,2-b]fluorenes: Synthesis, Crystal Structures, and Photophysical Properties
Chase, D. T.; Fix, A. G.; Rose, B. D.; Weber, C. D.; Nobusue, S.; Stockwell, C. E.; Zakharov, L. N.; Lonergan, M. C.; Haley, M. M. Angew. Chem. Int. Ed. 2011, 50, 11103–11106.
DOI: 10.1002/anie.201104797
Synthesis, Crystal Structures, and Photophysical Properties of Electron-Accepting Diethynylindenofluorenediones
Rose, B. D.; Chase, D. T.; Weber, C. D.; Zakharov, L. N.; Lonergan, M. C.; Haley, M. M. Org. Lett. 2011, 13, 2106–2109.
DOI: 10.1021/ol200525g
Indeno[1,2-b]fluorenes: Fully Conjugated Antiaromatic Analogues of Acenes
Chase, D. T.; Rose, B. D.; McClintock, S. P.; Zakharov, L. N.; Haley, M. M. Angew. Chem. Int. Ed. 2011, 50, 1127–1130.
DOI: 10.1002/anie.201006312